How to calculate the M and N of solutions after mixing 100ml 0.2M H2SO4 with 50ml of 0.1M HCl - Quora

10 ml of 0.1 M H2SO4 is mixed with 20 ml of 0.1 MKOH, the pH of resulting solution will be(a) 0(b) 7(c) 2(d) - Brainly.in
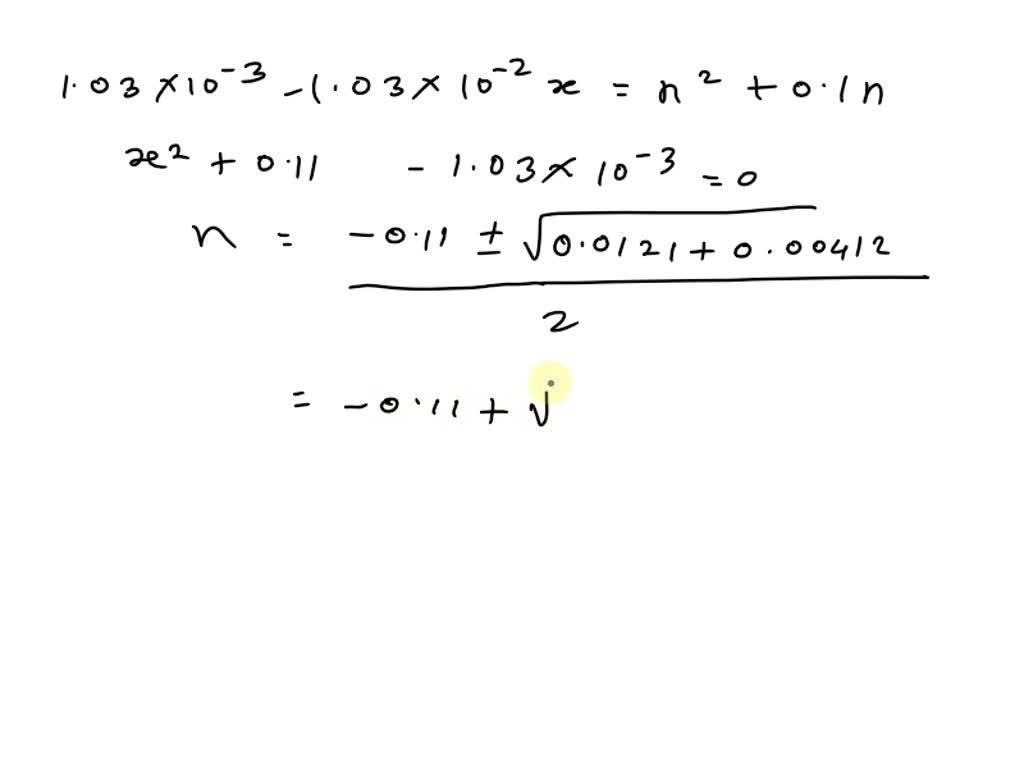
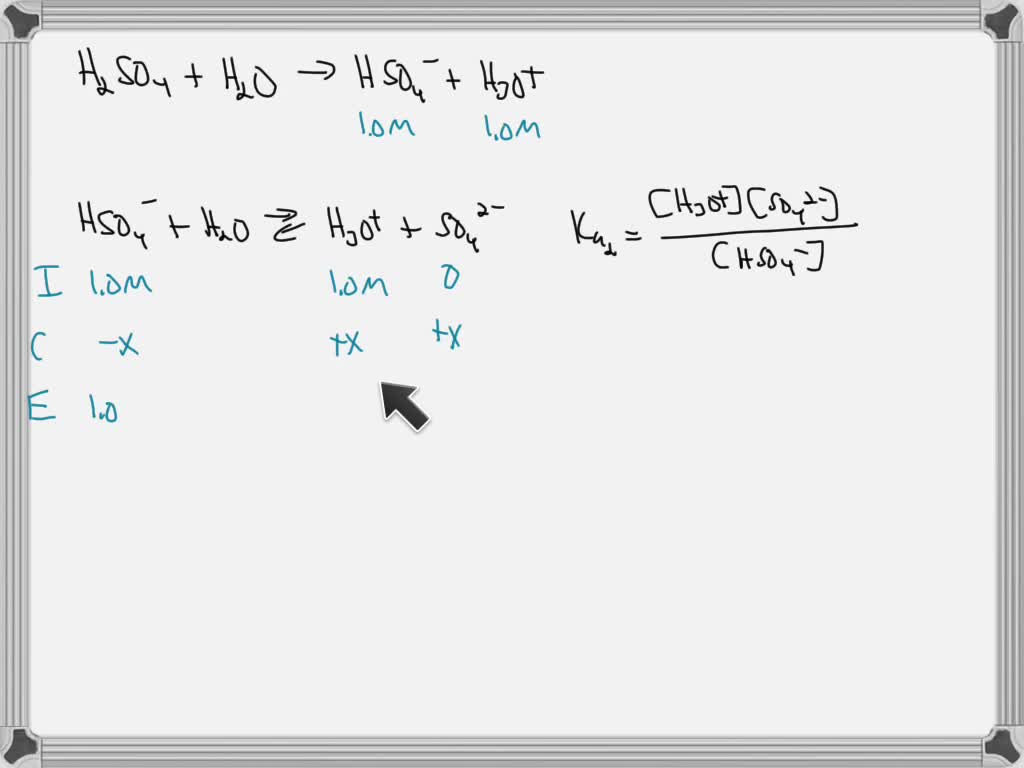
SOLVED: A. Calculate the pH of 0.1 M H2SO4 solution in water. Ka2 = 1.03×10^-2 B. What will be the pH of 0.1 M H2SO4 solution in 0.1 M K2SO4 solution? Use
What is the pH of solution made by mixing equal volumes of 0.1 M-H2SO4, 0.1 M-HCl and 0.1 M-HNO3? - Quora
Consider the following statements (a) The pH of a mixture containing 400 mL of 0.1 M H2SO4 - Sarthaks eConnect | Largest Online Education Community
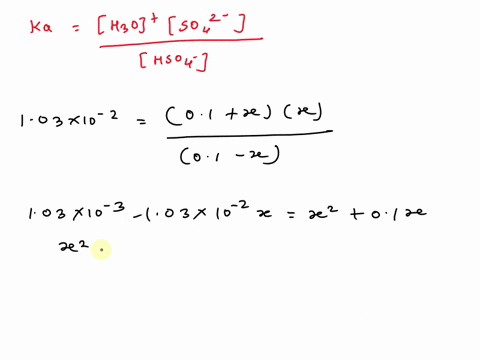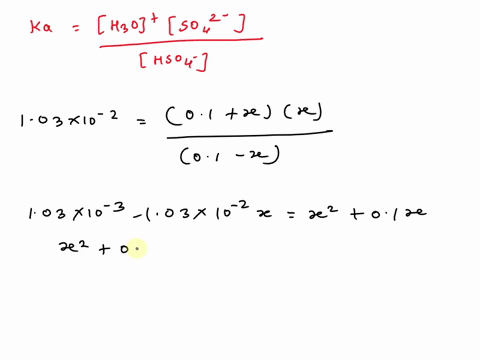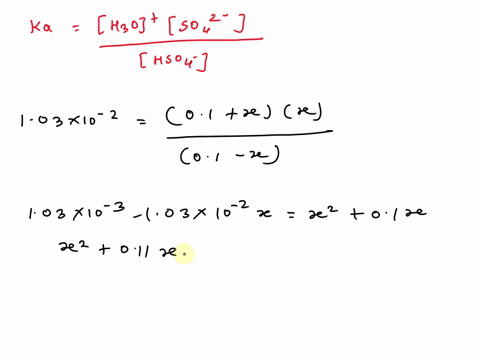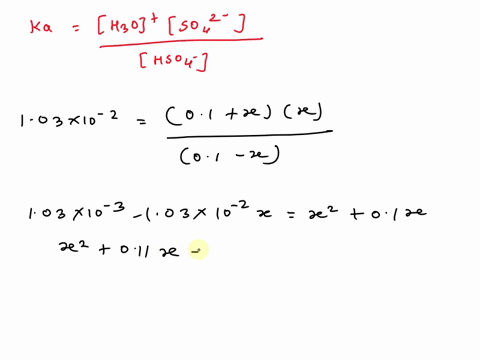
SOLVED: A. Calculate the pH of 0.1 M H2SO4 solution in water. Ka2 = 1.03×10^-2 B. What will be the pH of 0.1 M H2SO4 solution in 0.1 M K2SO4 solution? Use
100 ml , 0.5(M) H2SO4 solution is completely neutralised by 50ml 0.1(M) NaOH solution and Xml 0.1(M) Ca(OH)2 solution . Find value of x.

200ml N/20 HNO3, 100ml N/20 H2SO4, 300ml M/30 HCL, 50ml 0.1M Ba(OH)2 and 250 ml M/10 NaCl solutions were mixed then value of pH of resultant solution is:- (1) 1.65. (2) 2.0 (3). 1.78. (4) 0.16? - EduRev NEET Question
What is the pH of the resulting solution when equal volumes of 0.01 m H2SO4 and 0.1 m HCl are mixed (log 3 = 0.477)? - Quora